Recently, Professor Sun Jinpeng's research team of the school of basic medicine, Professor Wang Jiangyun's research team of the Institute of biophysics of the Chinese Academy of Sciences and Professor Chen Chunlai's research team of Tsinghua University jointly published an online publication entitled "single molecular fret and conformal analysis of" in Chemical Science (IF = 9.8 in 2021, chemical zone 1) β- Arrestin-1 "through genetic code expansion and Se click reaction". He Qingtao, a doctoral student of the school of basic medicine of Shandong University, Han Mingjie, an assistant researcher of Tianjin Institute of industrial biotechnology, Chinese Academy of Sciences, and Yang mengiridium, a doctor of Tsinghua University, are the co first authors of this paper. Professor Sun Jinpeng, researcher Wang Jiangyun and Professor Chen Chunlai are the co corresponding authors of this paper.
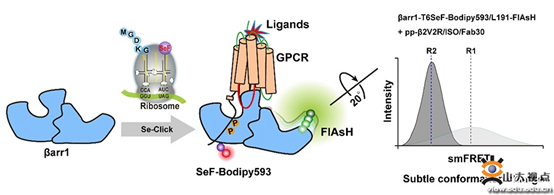
In order to capture GPCR and regulate the conformational state distribution of downstream protein Arrestin, the research team used the gene code extension technology to select and specifically insert the non-natural amino acid SeF into the specific site of Arrestin, and then specifically label the fluorescent dye Bodipy593 into SeF.In this way, the targeted marker of Bodipy593 on Arrestin was used as the receptor. Meanwhile, the research team inserted CCPGCC into the specific site of Arrestin to mark Flash-EDT2 as the donor. With the help of TIRF fluorescence microscope platform of Tsinghua University, Phosphorylated GPCR by single-molecule FRET assay regulates the distribution of different conformation states of Arrestin.
In this study, the team innovatively utilized the gene codon extension technology to integrate the selenium probe SeF into the specific site of Arrestin, and realized the efficient and specific labeling of Bodipy593 through substitution reaction. Compared with Bodipy593, the labeled SEF-Bodipy593 showed a 47 nm red shift.It also reduces the background noise of the signal. Compared with the traditional cy3-Cy5 labeling method, all Cysteine mutations in Arrestin protein were avoided. Sef-bodipy593 and FlAsH realized the specific labeling of proteins by dye with shorter Linker.It is easier to accurately detect the small conformational changes and state distribution of Arrestin before and after activation.
Paper link: https://doi.org/10.1039/D1SC02653D