On August 5, the research team of Professor Sun Jinpeng of the school of basic medicine, together with the research team of Professor Zhang Jian and Professor Lu Shaoyong of the school of medicine of Shanghai Jiaotong University and Ningxia Medical University, published online in the journal Nature communications (2021 if = 14.919) “Activation pathway of a G protein-coupled receptor uncovers conformational intermediates as targets for allosteric drug design” He Xinheng, a doctoral student of Shanghai Institute of pharmacy, Chinese Academy of Sciences, Yang Zhao, an assistant researcher of School of basic medicine, Shandong University, and Zhou Shuhua, a master's student, are the co first authors of this paper, and Professor Sun Jinpeng, Professor Zhang Jian and Professor Lu Shaoyong are the co corresponding authors.
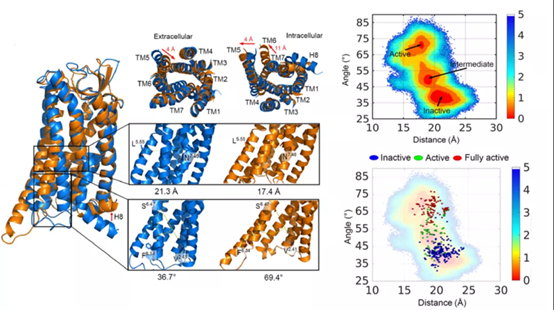
In this study, angiotensin receptor AT1R was used as model GPCR.AT1R is an important drug target in cardiovascular and renal diseases, and its overactivation can lead to diseases such as hypertension and aneurysm.Antagonistic Gq and Arrestin activity of AT1R have significant effects on these two diseases, respectively, based on which clinical sartan drugs have been developed. This study from the recently published AT1R inactivation and activation states of crystal structure, identified the inactivation and activation states between the minimum energy path, mapped the AT1R activation process of the potential energy surface, the stability of intermediates, using markov model reveals the dynamic character of conformational state conversion between, reveal the conformation transition of the key amino acids conversion changes, It provides a basis for the discovery of implicit allosteric sites.The P6 region between TM1-TM7 and H8 is predicted and verified as a potential allosteric site unique to intermediate states. This site appears with the up-migration of H8 during activation and can co-regulate G protein and β -Arrestin signals, indicating its important role in the conformational change of AT1R receptor. This work developed a new strategy for identifying implicit allosteric mechanisms and sites, and provided new ideas for the design of AT1R allosteric agonists.
Paper link: http://www.nature.com/articles/s41467-021-25020-9